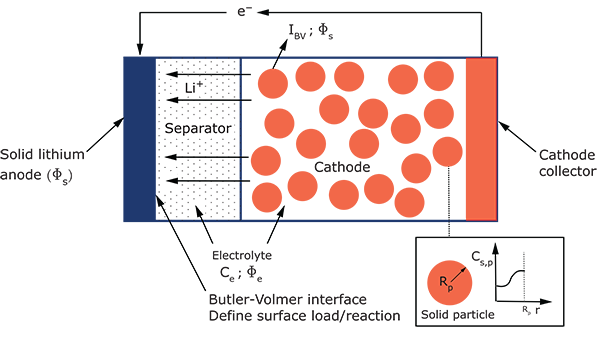
The governing equations for the thermal-electrochemical process are based on the
porous electrode theory and are described in detail in Governing Equations
in Coupled Thermal-Electrochemical Analysis. At the interface between the
separator and the solid lithium anode, a charge-transfer reaction occurs following a
Butler-Volmer kinetics that is expressed using the following equations:
Here,
represents lithium in the solid electrode that, through the
deintercalation reaction, transforms into a lithium ion in the electrolyte,
, plus an electron,
, and leaves behind a vacancy (or intercalation site) in the solid,
.
The general form of the Butler-Volmer current expression is:
where
and
are the exchange current density and overpotential, respectively.
The overpotential,
, is defined as:
In the above equations,
-
- is Faraday's constant;
-
- is the gas constant;
-
- is the charge number of the lithium ion battery;
-
- is temperature;
-
- is the absolute zero temperature;
-
- are the cathodic and anodic transfer coefficients,
respectively;
-
- is the reference value of lithium ion concentration in the
electrolyte;
-
- is the ion concentration in the electrolyte from the separator
side;
-
- is the open circuit potential (OCP) as a function of
. The typical value in a solid anode is zero.
-
- is the electric potential from the anode side; and
-
- is the fluid electric potential from the separator
side.
The electrochemical interaction at the solid electrode-separator interface is
represented on the solid electrode side as a surface current density of magnitude
.
represents a dimensionless “surface factor” that captures the
effects of surface irregularities that can result in the availability of a larger
surface area over which the electrochemical interaction happens. On the separator
side of the interface, the electrochemical interaction results in two separate
surface fluxes, both applied on the electrolyte phase: a fluid current density of
magnitude
and an ion flux of magnitude
, where
is the Faraday constant. For more details on the electrochemical
governing equation, see Conduction of Electrons in the Solid Phase and Diffusion and Migration of Lithium Ions in the Liquid Phase. However, an important distinction between the referenced governing equation
sections and the current surface interaction load is that the conduction and the
diffusion/migration equations in the referenced sections treat the effects of the
Butler-Volmer kinetics as a volumetric source. Here, they contribute as surface
fluxes for the solid lithium electrode battery.